Pesticides are a group of chemicals that are used by man to control a wide variety of pests from weeds to insects and animals.
Herbicides are designed to kill plants, insecticides kill insects, fungicides kill fungal infections, acaracides kill spiders, molluscicides kill snails and slugs, rodenticides kill rats and mice, and many other, or similar, chemicals are used to treat animals for parasites, such as warblecides that kill the grubs of the Warble Fly within the body of cattle. Although some of these chemicals act specifically against one species most have equally damaging effects in non-target species (those not intended to be harmed), and that includes man.
As examples; fungicides can be harmful to bees; insecticides can damage the reproduction and food supply of birds; and molluscicides have killed cattle and dogs.
There is little doubt that these chemicals have reduced suffering and increased yields when used correctly but there is also certainty that those same chemicals have been implicated in serious adverse effects both in man and in the environment.
Thousands of chemicals and chemical formulations are involved and most of them are poisonous to organisms other than those that man intends to kill. Only very few of those chemicals have been properly tested and even fewer have been assessed for effects in mixtures or combined exposures even though Man himself is one of those non-target organisms.
Human exposures to pesticides can result from lack of protection during use, residues in treated foods (and that includes all diets be they vegetarian or meat inclusive), and from contaminated air, water and clothing. In addition the chemicals can be tracked into homes on footwear or by contaminated pets.
Some pesticides are produced from nature and normally break down quickly in the environment and are therefore short-lived, while others are man-made with the intent to make them long acting. They are therefore more persistent in the environment and pose special dangers.
All of the products are specifically designed to kill and the most successful kill very efficiently.
Formulations and Mixtures
Very few pesticides contain only one ingredient.
The active ingredients (the chemical relied upon for the desired action) may take the form of powders or liquids that are mixed with substances that may be inert, having little or no pesticide action, or they may have toxic actions of their own.
The various co-formulants in pesticides can increase toxicity of the active ingredients and many are extremely toxic in their own right. Chemicals in the formulations can be volatile, giving of toxic vapours, and some, like benzene, have been known to cause diseases such as leukaemia for at least 50 years.1
Others, such as naphtha, have insecticidal properties and were actually used for that purpose many years ago.
It has been admitted that some co-formulants are present as a means to protect the active ingredient or to enhance the effectiveness and prolong the activity of the formulations.2
Some pesticides or their solvents are known to trigger chemical sensitivity reactions, known to sufferers as Multiple Chemical Sensitivity, in which the slightest amount of chemical can trigger debilitating and in some cases life-threatening reactions.
The Military of the USA have produced a valuable paper entitled "Chemical Exposure Guidelines for Deployed Military Personnel"
http://chppm-www.apgea.army.mil/desp/pages/samp_doc/TG230/TG230%202002.pdf
Pesticides and many of the solvents used in the pesticide formulations, their health impacts, and their carcinogenic potential are listed in detail.
In the light of current official denials it makes interesting reading.
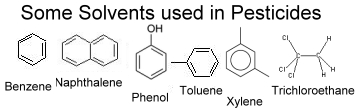
Benzene – a volatile solvent that forms the basis for a range of aromatic carbon ring based chemicals. It is a good solvent for fats and its vapour is toxic. Benzene acts as a narcotic, is an irritant, and a bone marrow depressant with anaesthetic action. The symptoms of benzene poisoning include burning, sore skin with redness, swelling and blistering, dizziness, excitement, headache, nausea, loss of appetite and weight, fatigue, weakness, nose bleeds and in severe cases, coma. Benzene is reported to be stored in the bone marrow for months after exposure1 and has been recognised as a cause of bone marrow disease and leukaemia for much of the last century.3 Benzenes were used for decades in insecticide formulations added to human food grains. Only in recent years has the European Union ordered a reduction in the Benzene content of pesticides.
Toluene – is a homologue of Benzene (it has a similar structure) and has similar narcotic and anaesthetic action. As with Benzene its vapours are toxic and the symptoms include irritation of mucous membranes, vertigo, loss of balance, headache, respiratory depression and coma.
Naphthalene – has a penetrating tarry (Mothball) smell. It is toxic and has been shown to cause cataracts when the eyes are exposed to the chemical. Naphthalene damages or destroys red blood cells. Some forms of naphthalene are known liver and nervous system poisons, affecting both the central and peripheral nervous systems. Symptoms include fatigue, loss of appetite, restlessness, pale skin, nausea, vomiting, diarrhoea, blood in urine and yellowing of skin. It is classified as a possible carcinogen and there are reports that haemolytic anaemia can result from inhalatory maternal exposure during pregnancy or in exposed infants. Naphthalene is more reactive than Benzene and has been used historically as an insecticide in its own right.
Phenol – is formed from Benzene, is soluble in water and is also toxic, with antiseptic and anaesthetic properties. Phenol is the base for industrial production of aspirin, weedkillers, and some synthetic resins.
Phenols can cause severe chemical burns on contact with skin. Phenols have been used as disinfectants and are considered to be one of the most dangerous constituents used in the Sheep Dips that caused illness in so many shepherds in the UK. Phenols are reported to have insecticidal properties. They penetrate the skin and are nervous system toxins.
Nazi scientists are reported to have used Phenol to kill prisoners by injection into the heart. 4
Xylene - is found in three forms, or isomers, all of which are homologues of Benzene. The vapour from xylene is toxic and, like Benzene the chemical damages the bone marrow, although Benzene, is the more dangerous product. The symptoms include weakness, fatigue, headaches, irritability, sleeplessness, loss of memory, tinnitus (ringing in the ears), dizziness, “drunkenness”, shivering, shortness of breath, sweet taste in the mouth, nausea, irritation of the eyes and nose, and in severe cases, coma.
Other derivatives of Benzene used as solvents – are reported to have direct central nervous system toxicity, anaesthetic effects, impair the oxygen carrying capacity of blood, damage the blood itself, suppress bone marrow, irritate the respiratory and gastro-intestinal tracts, trigger allergic effects, including sensitivity, and have carcinogenic effects such as cancer of the urinary tract and the blood.
1,1,1 Trichloroethane – is one of a group of organic solvents called chlorinated hydrocarbons and is also known by other names including methyl chloroform. Usually in liquid form it evaporates quickly, with vapours heavier than air. The USA stopped producing the chemical in 1996 because once in the atmosphere it can remain there for 6 years and adversely affects the Ozone Layer. The solvent can damage the skin, eyes, liver, kidneys, and nervous system.
Exposure is usually by inhalation but skin contact may cause irritation and there is evidence that liver and kidney damage may also result. Symptoms include headache, nausea, dizziness, irritability, weakness, slurred speech, loss of balance, low blood pressure, and in severe cases fatigue, impaired coordination, concentration loss, personality changes, palpitations and even death. No studies have been done to confirm carcinogenicity or effects on reproduction in exposed people but it is known that the solvent can affect the foetus and contaminate breast milk.
There have been no studies in people to determine any harmful effects from eating food or drinking water contaminated with this solvent and yet it has been a major constituent in grain store insecticides that are added to the grain used for human consumption
Often even single "active ingredients" may themselves contain "contaminants" or "impurities" which can be exceedingly dangerous even in very small amounts. 5
Dioxin is probably the most famous contaminant and has been involved in many incidents where serious harm has been caused to human health but there are many others, including TEPP, an organophosphate known to have killed a herd of cows within an hour of skin applications.6
The rules of "Commercial Confidentiality" prevent members of the public from obtaining the full details of chemical formulations or contaminants. Those who support such secrecy will tell us that this is to protect the manufacturers but in truth manufacturers of chemicals protect formulations through the patent system and they can determine formulation content with modern methods not available to the public.
The suspicion is then that the secrecy is to protect the manufacturers from any members of the public who believe themselves to be harmed by the chemicals.
Without that vital knowledge those who believe themselves to have been poisoned have difficulty proving that the ingredients caused the harm to their health.
It is well known that some chemicals enhance toxicity of substances that would not be toxic alone. The scientists refer to this process as "Potentiation" which is a special form of synergistic effect. "Synergy" occurs when chemicals combine with increasing effect.
Scientists tell us that in the majority of cases synergistic reactions are merely additive and they tend to ignore or deny the more dangerous "potentiation".
So why are pesticides dangerous?
Although these chemicals are designed to kill pests, parasites and weeds, the life force in all these living organisms depend on very similar processes and naturally occurring chemicals.
As a result a chemical that is toxic to a pest is very likely to be also potentially harmful to man because the mechanisms targeted are similar.
Furthermore a pesticide application to a pest or weed is likely to be fatal, as intended, but man will be exposed to non-fatal doses of a host of chemicals and each may react with the other to form more dangerous substances or cause the failure of different mechanisms within the human body.
It has been known for decades that if a pesticide worker has been harmed by exposure to one group of chemicals then it is extremely dangerous for that worker to move to using another chemical that has a different target in the body. 7
The same is true when humans absorb a variety of pesticides in their food, water, air, and from the things they touch or contaminated clothing that they may wear.
It should be remembered that many pesticides are cumulative in action and so every exposure adds to the last either as a chemical build-up or as the result of increasingly damaging effects.
Many poisons are also irreversible in effect, which means that once the damage is done it cannot be undone.
As a result every exposure, no matter how small, increases the damage done in the body.
Those pesticides that combine both actions are both cumulative and irreversible in effect and can be extremely dangerous. Organophosphates and pyrethroids 8 are two such chemicals and this is why a UK government committee ordered that organophosphates should be labelled as "DEADLY POISONS" in the 1950s. 9
Scientists in the USA declared in the 1990s that even when children ate food below the maximum "safe" residue levels for individual insecticides their total consumption of residues could reach dangerous levels. 10 Those dietary intakes fail to take account of other exposure routes that could further raise the intake levels of pesticides or other environmental chemicals.
The human body depends on the proper functioning of every process and they are all kept in balance in a very complex and interdependent system. The controls are all chemicals and pesticides are designed to target those processes and to make them fail.
Such failures can induce vitamin, mineral and hormone deficiencies, even when there are no dietary factors involved, and the resulting illnesses can be almost indistinguishable from those of "natural causes".
Multiple Exposures Are Dangerous
Each poison targets different enzymes and processes in the body and each poison therefore adds to the cumulative damage. In this way repeated exposures, even at low doses, can cause permanent changes and long-term effects at levels far below those that can be determined in a doctor's surgery or a science laboratory. When suffering from those effects further exposures to that or any other should be avoided until recovery is complete. 7 In the real world outside of the laboratory mixtures of pesticides are more frequently used than single applications. The effects of these multiple exposures on individuals already suffering the effects of pesticides, or those on medication, or who are already frail or unwell, can be deadly.
What makes a poison?
Those who would try and convince the world that pesticides are no more dangerous than salt would have us believe that in all cases it is the dose that makes the poison. Although that may be true with some chemicals it is by no means true of all.
Much depends on the health or genetic make-up of the individual person and there have been many cases where thoroughly tested medicines have caused fatal reactions after the administration of normally safe doses. In such events the danger may well be not from the toxin but from the reaction of the human body to what could be infinitesimally small amounts of a toxin. Such deadly reactions have been recognised for many years in latex or nut allergy where reactions to the smallest levels have caused death in the exposed individual.
Many chemicals can become extremely dangerous if heated and it is known that for some contact with the skin after heating in fire can result in the need for amputation because of the toxic effect on flesh. (Insurance assessors have warned of just such dangers from electrical components in vehicles that have been destroyed by engine fires)
For many Organophosphates the true toxic potential is not realised until the chemical is broken down in the body of the victim where the metabolic processes themselves alter the original chemical and form even more harmful chemicals that attack from within.12
The key to toxicity of any chemical is NOT the chemical formulation but it is the molecular shape of the toxin.13
This directly counters the "Dose makes the poison" argument.
In January 2002 the UK regulatory body, the Pesticide Safety Directorate, released a safety evaluation paper in which they referred directly to the dangers of chemical isomers, which are chemicals that have the exact same formula to those considered "safe" but they have a different molecular shape and can be highly toxic as a result. This is well known in the pharmaceutical industry and is used as a means to make drugs more effective.
All processes in the body depend on the basic and relatively simple, but highly efficient use of, specifically shaped chemicals that fit only in the intended receptor sites.
The following illustrates just a few of the hundreds of potential substances that may be adversely affected by chemical toxins. By far the majority are dependent on or are themselves compounds that contain oxygen and phosphorus bonds and are therefore extremely susceptible to substitution in vital processes by the organophosphorus compounds. Those OP chemicals can break down to release stored energy, often as heat, and harm further essential processes. Once the vital building processes are damaged the result can be abnormal protein formation and imbalance in vital substrates within the body, including hormones, the action of which is dependent on Cyclic AMP (cyclic adenosine monophosphate), which is in turn dependent on ATP/ADP energy transfer.
Once bound to the correct receptors the next process can take place and other chemicals may be added to form whatever product is required by the particular body function on which the process depends. Obviously that is a very simplistic explanation and in reality there are complex interactions and back-up processes that protect from damage to those systems - up to a point.
Poisons contain chemicals that are so similar in molecular shape to those vital natural chemicals that they can also bond to key receptors.

Poisons cause harm because they are not the correct chemicals and so, once bonded to the receptors, the vital processes depending on the correct bonds fail and that in turn leads to the failure of other processes and eventually, in severe poisoning cases, organ failure and even death may follow.
HOWEVER, a very recent case in the UK demonstrates with certainty that even at very low doses of certain toxins extensive and serious multiple organ failure can result.
Much media coverage has been given to a drug trial involving a new pharmaceutical product that had supposedly been tested in animals with no obvious, or at least admitted, adverse effects other than swelling of the neck and lymph nodes in monkeys.14
The drug in question appears to have been a product of genetic engineering with the hope that it would offer a treatment for rheumatoid arthritis, leukaemia, and multiple sclerosis, and it was given the name TGN 1412
Far from being administered at a high dose the experiment is reported to have involved the injection into paid volunteers of a very low dose of 1/500th of that used on animals.
Despite the low dose it was reported that patients suffered serious adverse effects within 5 minutes of the first injection - a further 5 doses had been planned. Patients reported serious headaches and back pain and one was described as being bloated "like the elephant man". All those injected with the drug required artificial maintenance of the vital body functions, including dialysis due to kidney failure. One was reported to be in a coma that was likely to last for up to a year.
In 2005 the Royal Commission on Environmental Pollution in the UK suggested that more animal testing was required to ensure that pesticides presented no risk to the public. The problem is that not all test animals are equal. There are numerous varieties that are bred to be more or less susceptible to the induced effects during trials. In October 2000 the UK regulators actually wrote to chemical companies with the helpful suggestion that they should use more resistant test animals because too many were dying before the testing procedures had been completed.
The effects of the drug TGN 1412 have demonstrated not only that the ancient wisdom 15 that it is the "dose that makes the poison" is not valid for modern man-made toxins but also that animal testing does not offer a certain indication of safety if humans are exposed. There must also be questions regarding the safety of genetic modification of medicines and a need for much greater understanding of the way processes interact within the body.
The most important effect of chemicals is the way in which the human body reacts to the presence of the chemical - at whatever dose.
The problem for the regulatory agencies is that for many chemicals the effects may well be different both in action and severity in individuals but they have a duty to ensure that none of us is harmed.
Pesticides are designed to target vital systems within the body and the closer in molecular structure they are to vital natural substances on which life depends the more effective they will be.
Natural Disease or Poisoning?
All poisons mimic natural disease. Both involve the failure of essential processes at cellular level.
Organophosphates are particularly dangerous because the body employs phosphorus-based natural substances at many stages in thousands of processes vital to life. Most important of these is the energy transport system where oxygen and phosphorus atoms are combined as part of the respiratory process, which is controlled by the mitochondria. Energy transport and storage is a complex process involving a variety of different systems in order to ensure a reliable source of power for muscles, the brain and all bodily functions, including the immune system, protein formation, and cell reproduction.
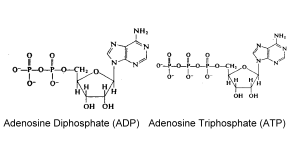
The ultimate source of energy in all living things is the breakdown of food molecules and the energy obtained from that process is conserved in a chemical called adenosine triphosphate. That chemical is formed by the addition of phosphorus and oxygen atoms to the chemical adenosine diphosphate. This is known as oxidative phosphorylation and a study of pesticides and their actions will show that many target this energy production process, which is vital to life and all processes in the body.
Scientists have known for decades that organophosphates interfere with that process and prevent the efficient transfer of energy, resulting in lack of oxygen at essential points. 12
This may well explain the fatigue seen in ME (Myalgic encephalomyelitis) and CFS (Chronic Fatigue Syndrome) cases and toxicologists confirmed in the early 1990s that CFS was known even then to be caused by pesticides or viruses.
Interestingly this is now denied by regulatory authorities in the UK.
More importantly so many processes in the body depend on these natural "organophosphate" energy suppliers that when that process is damaged the symptoms that result can be different and wide-ranging between individuals. Not only does OP poisoning appear to affect people who are especially energetic in their work but also there are suggestions from sufferers that they are more affected on the dominant sides of their bodies. Perhaps this indicates that poison take-up is related to the ATP/ADP process and is greater in the more active systems?
ME/CFS, asthma, cardio-respiratory illness, auto-immune diseases, Motor Neurone, Multiple Sclerosis, Alzheimer's, Parkinson's, Schizophrenia, cancers, diabetes, and bone deterioration, have all been linked by scientists at some point to various chemical exposures.
For at least 50 years the regulatory authorities have called for more research and yet they always seem to find some reason or another to dismiss the findings of scientists who discover links between pesticides and disease. Even government funded papers that clearly admit to knowing the long-term effects of pesticides on human health are ignored, 3,16 as has been the military experience of chemical trials and published work of brave scientists who poisoned themselves in their efforts to properly understand the problem. 1
Definition of a Poison.
A poison is a substance that can seriously adversely affect health or can destroy living organisms by actions on the tissues and life supporting systems within the organism, by contact, ingestion, inhalation, or by entering the circulatory systems.
When applied to surfaces they may be absorbed into the body where they may undergo changes that may increase their toxic effect.
The legal definition of a poison is of a substance which, when administered, is injurious to health or life.
Poisons may be corrosive, or irritants, and most have a systemic action within the body in addition to any obvious localised effects.
In all poisonings the harmful effects eventually result in damage to vital organs such as the liver, kidneys, lungs, heart, and the immune, endocrine, and nervous systems.
The difference between a "toxic dose" and a "lethal dose" may depend as much on the individual as the actual toxin in question. Some people are far more susceptible than others, especially the sick, elderly, those taking medication, and obviously the young and unborn, whose protective immune systems are not yet fully formed.
It has been known for 50 years that even commonly used drugs such as aspirin can cause serious adverse reactions in susceptible people.
The route into the body taken by the poison is also important, as is the solubility of the poison itself.
Most rapid actions are found when the poison is inhaled as a gas or vapour or is injected into the blood stream while dermal and ingested poisons can act more slowly, depending on the toxin. Some of the more dangerous organophosphates were developed specifically because they readily penetrate the skin and enter the blood stream, rendering the entire body toxic to the target organism. Warblecides are such chemicals and government officials in the UK advised farmers that skin applications of these chemicals had the same effect as injecting them directly into the blood stream. Quote “this will be absorbed in exactly the same way that an injection would be” 17
Some chemicals are reasonably safe on their own but can become lethal in combination or when changed within the body.
Many poisons such as lead, mercury and carbon monoxide tend to accumulate in the body until levels are reached when they trigger poisoning symptoms. With others, such as the organophosphates, the damage caused by the poison accumulates with every exposure until the damage is sufficient to trigger symptoms. In such cases the individuals may have no idea that they are being poisoned until it is too late to act.
A major company that supplies veterinary medicines and advice to the profession lists organochlorines, organophosphates, and pyrethroids, under the heading of "Chemotherapeutic Chemicals". 18
Diagnosis of Poisoning.
Because the symptoms of poisoning closely resemble those of natural disease diagnosis of poisoning is often very difficult, especially if the patient does not know anything of the exposure to poisons.
Arsenic poisoning for example may produce similar symptoms to those of food poisoning.
Organophosphates will produce symptoms very similar to influenza.
Dinitro compounds can turn the skin yellow as in jaundice.
Diagnosis requires evidence and to obtain that evidence requires the unbiased skills of the medical profession and direct access to diagnostic equipment, which is often complex and extremely expensive.
Evidence of poisoning 13 includes:
- The symptoms follow exposure to the toxin.
- The symptoms may be similar to others exposed to the same toxin.
- There may be evidence of the presence of the toxin in the body fluids.
- In fatal cases there may be post-mortem evidence.
(Not always easy to determine as some poisons may induce a delayed effect, sometimes weeks after exposure)
(But this may depend on the level of exposure, individual susceptibility, and the specific formulation in which the poison is incorporated.)
(Blood, urine, faeces, sweat, saliva, vomit or stomach contents.)
(Skin, lungs, liver, kidneys, heart, brain, fat, viscera may show evidence consistent with poisoning.)
The Symptoms induced by exposure to pesticides and the co-formulants can be "Non-Specific" in that they are not caused only by that chemical or group of chemicals. Such "non-specific" symptoms may be flu-like symptoms including dizziness, malaise, coughs, sore throat, headaches, rashes or reddened skin, sweating, gastrointestinal symptoms, nausea, vomiting, blurred vision, seizures or strokes, heart rate abnormalities and the effects of the odours of the vapours given off - which are not necessarily merely the toxicological effects of the active ingredient alone.
The part played by solvents in the causation of symptoms is often overlooked.
It is of interest that many of the scientists involved in the study and regulation of pesticides dismiss the effects of odours and vapours as symptoms that have no toxicological basis with the suggestion that any symptoms are the result of "hysteria" or an over-active imagination. 19 What they forget is that some pesticides are specifically designed to act by vapour release and in many cases the symptoms are experienced before the sufferer is even aware of the presence of the toxin. They also forget that the olfactory passages (of the nose) are a direct route to the brain and that very small exposure to toxins may damage the essential processes within the brain.
Despite the difficulty in obtaining evidence to support their allegations that the symptoms are the result of the "perception of risk" these individuals still try to accuse the sufferers of suffering from mental illness. 20
Pesticide symptoms can mimic those of other illnesses and the literature shows that those illnesses include Asthma, Influenza, Heart Attack, Brain haemorrhage, Diabetes, Gastroenteritis,Heat Stroke, Pneumonia, Viral infection, Meningitis, Bronchitis, Epilepsy, Alcoholism, Parkinson’s Disease, Multiple Sclerosis, Alzheimer’s Disease, and even Heat Stroke.
Some of the drugs commonly used to treat those illnesses can be extremely dangerous in poisoning cases and the idea that there are antidotes to poisons is not always supported in fact. Most treatments are administered to block receptors and reduce the harm done or to provide assistance to the body by eliminating the poison and treating the symptoms appropriately, often with administration of oxygen.
Drugs that may be dangerous for those who have been poisoned include the contraindicated treatments
Theophylline and Aminophylline, which are used to treat asthma, certain types of antibiotics and antidepressants, some anaesthetics, and painkillers such as morphine. Treatments such as exercise, and a host of drugs that act on the same biological pathways as pesticides, may also be dangerous in poisoning cases.In some cases oxygen itself can worsen symptoms.
Children and the unborn are at greatest risk from pesticides because their immune, endocrine and nervous systems are still forming and they suffer a greater level of exposure relative to their weight. Recent research from Liverpool University in the UK suggests that the health of children may be harmed by residue levels as low as parts per trillion. 31
Children are also more vulnerable because they are unaware of any risk and they often crawl or play in treated areas without understanding the sort of precautions they should take. Children tend to have more hand to mouth contact and there is therefore a greater risk of ingesting pesticide residues.
Children are more vulnerable than adults and they need greater protection.
Common pesticides, their actions, symptoms, and treatments.
(It is advised that expert medical advice is sought in all cases.)
Arsenical pesticides
Inhibit enzymes and affect the circulatory system damaging blood vessels. It is also reported that arsenical compounds inhibit oxidative phosphorylation. These compounds are recognised for their ability to induce cancer and allergic reactions. Lung cancer due to arsenic is no different to other lung cancers.
Confirmation of diagnosis is by analysis of vomit or faeces, as arsenicals do not remain in blood for long.
Ingestion is the main route as absorption through the skin and by respiration is slow.
They cause abdominal pain and vomiting and diarrhoea on ingestion, with effects on the heart and kidneys.
Chronic poisoning causes weight loss, anorexia, peripheral neuropathy and skin problems. Lung cancer links are reported.
Treatment includes intravenous fluids, dimercaprol injection, artificial respiration and cardiac resuscitation.
Bromoxynil and pesticides with similar action
Adversely affect oxidative phosphorylation, stimulating the metabolism of the cells.
Skin contamination is the main exposure route but inhalation also carries risk and they are highly toxic if ingested.
Confirmation of diagnosis is by analysing blood plasma or serum for the presence of the chemical.
Fatalities have been recorded in hot climates but the action of the poison is said to be reversible in most cases. Symptoms include fatigue, excessive sweating and thirst, which may easily be confused with heat or work stress. Increasing anxiety and restlessness, respiratory distress, rapid heart beat, sleeplessness and loss of weight may accompany serious poisonings.
Treatment includes artificial respiration, and measures to maintain heart rate and control convulsions.
There is no specific treatment but rest is essential and oxygen and intravenous fluids may be required.
Carbamates
Like Organophosphates these chemicals inhibit the enzyme acetylcholinesterase (AChE), which is essential for the correct transmission of nerve signals and is found in the blood, brain and other tissues. Although these chemicals act quicker than organophosphates the bond with the enzyme is weaker with carbamates and so the damage caused is reversible and recovery is quicker.
Inhalation, ingestion, and skin contamination, are all important routes of entry into the body.
Symptoms include headache, nausea, respiratory restriction, cough, muscle twitches, weakness, sweating, excessive saliva and bronchial secretions, with low blood pressure, heart rate irregularities, incontinence, and mental confusion. In serious cases death may follow respiratory and heart failure.
Symptoms normally cease within 24 hours.
Treatment is as an emergency and extra care should be taken to ensure that stomach contents do not enter the lungs because of the presence of solvents. Atropine is regarded as an antidote and repeated injections may be necessary. Oxygen, control of convulsions and efforts to maintain the heart may be required and all vital signs should be monitored.
It is considered that measurement of cholinesterase activity is unlikely to be helpful in carbamate poisoning because recovery is normally rapid.
Chlorphenoxy Compounds
Are irritating to the skin and mucous membranes and are known to cause acne-like skin conditions. They are metabolic and nervous system toxins, which have been reported to cause peripheral neuropathy in severe poisonings and functional disturbances in the brain.
These chemicals can be toxic via the skin, by inhalation, and by ingestion.
Symptoms include skin irritation, burning sensations in the throat and chest, cough, dizziness, pain in the chest and abdomen, headache, vomiting, mental confusion with bizarre behaviour, muscular stiffness or twitching, hyperventilation and raised body temperature. Body fluids become acidic, heart rate irregularities and liver cell damage may occur.
Muscle weakness can persist for months after poisoning.
Treatment includes removing the contamination, the use of activated charcoal or stomach emptying, intravenous fluids, and in severe cases action to correct the pH of the body fluids.
Given the correct equipment the presence of chlorophenoxy compounds may be measured in blood and urine. Body fluid pH measurement may also be performed but most of the compound will have been excreted within 72 hours of exposure.
Cyanides
Cyanides inhibit cytochrome oxidase, important in the mitochondria, and therefore block cellular respiration.
Exposure is normally to the Hydrogen cyanide gas given off by the products when exposed to moisture, although the chemicals have, on occasion, been swallowed.
Symptoms include anxiety, headache, dizziness, nausea, confusion, difficulty with breathing, low blood pressure, and heart rate irregularities. These lead to unconsciousness, cyanosis, and eventually death through respiratory and heart failure.
Oxygen and amyl nitrate are the first treatment choices followed in severe cases by the antidote (dicobalt edetate and dextrose, (or sodium nitrate) and sodium thiosulphate) if the expertise is available or otherwise as an emergency in hospital.
All those exposed should attend hospital where those less severely poisoned may only require Oxygen to fully recover.
Measurement of cyanide in blood samples is unreliable.
Dinitro compounds such as DNOC
Uncouple oxidative phosphorylation, increasing cellular energy transfer and stimulating the metabolism.
Exposure mostly by skin contamination but poisoning can also result from inhaling spray drift or dust.
Skin may be stained yellow but early symptoms are fatigue, sweating and thirst, fever, sleeplessness and loss of weight, all of which may be confused as heat or work stress. Continued exposure may result in respiratory, heart, and temperature control failures and death.
There is no specific treatment other than administration of oxygen and intravenous fluids with sponging with tepid water to overcome hyperthermia. Rest is essential.
The yellow colour in the blood and skin provide evidence of exposure and the chemical can be detected in the blood with the correct equipment.
Diquat
Irritant of mucous membranes, gastrointestinal tract and eyes.
Absorption, even on ingestion, is slow.
Symptoms include sore throat, dehydration, diarrhoea, and in serious cases there may be possible lesions in the brain, and renal failure.
Poisoning is uncommon and there is no specific antidote. Treatment is to replace lost fluids and prevent further absorption of the chemical from the digestive tract, possibly using activated charcoal.
Diquat can be measured in the urine or stomach contents given the availability of the correct testing equipment.
Metaldehyde
The toxic action of this chemical is not fully understood.
Exposure is normally to ingestion of the pellets used by gardeners and growers for slug control.
Symptoms include nausea, vomiting, abdominal pain and diarrhoea. More serious cases may suffer convulsions and unconsciousness.
Treatment includes maintaining the airway, control of convulsions, and correction of the pH balance in the body.
Methyl Bromide
Toxic action is not fully understood but it may bind to proteins, adversely affecting cell metabolism. It is also capable of crossing the blood brain barrier and causing central nervous system problems.
Exposure is normally by inhalation and, although it may burn skin, absorption via that route is poor.
Symptoms include delayed skin blisters, itching, fine rash and dry cracked skin. On inhalation the mucous membranes of eyes and respiratory tract can warn of the danger but exposure will cause headache, sore eyes, loss of appetite, abdominal discomfort, leading to paresthesia in lower limbs which can last for months, and possible kidney failure. In severe poisoning convulsions and unconsciousness may occur. Potentially fatal effects may be delayed and central nervous system symptoms include headache, focusing difficulties, ataxia (incoordination, clumsiness), drowsiness, incoherence and weakness.
Hospital emergency in severe cases where treatment is based on the symptoms exhibited. In severe cases of convulsions paralysis may have to be induced. CNS symptoms, including depression, irritability, impairment of concentration and visual problems may persist for months.
Measuring levels of bromide in the blood can confirm exposure.
Nicotine
Adversely affects the autonomic nervous system.
Absorption is rapid via skin, ingestion and by inhalation.
Extremely toxic and ingestion of 40 mg of pure nicotine can be fatal. Effects include nausea, dizziness, vomiting, respiratory effects, headache, rapid heart rate, sweating, increased saliva, low blood pressure, and in severe cases convulsions and coma.
Treatment for convulsions includes the use of diazepam and although there is no antidote, atropine can be used to control some features of poisoning. If death does not occur within 4 hours the patient usually recovers fully.
Blood plasma levels of nicotine can be measured with the correct equipment.
Organochlorine compounds
Alter the availability of sodium and potassium in nerve cells and act on enzymes. Some inhibit chloride transport via the GABA systems. Some are thought to be endocrine disruptors, altering the actions of hormones either directly or via their breakdown products. Many cancers have been shown to be associated with chemicals in this group that mimic natural hormones.
Organochlorines are absorbed through the skin, by ingestion and by inhalation.
Effects are on the central nervous system and the symptoms include depression, irritability, confusion chronic tiredness, headache, dizziness, and there have been links to aplastic anaemia, blood disorders, and breast cancer in some studies.
Treatment is to maintain vital signs and activated charcoal may be used when the poison has been ingested.
Organochlorines can be measured in the tissues, blood and plasma.
Organophosphorus Compounds
As with carbamates, organophosphorus compounds inhibit anticholinesterase (AChE) in the blood, brain, nerves and other tissues. They act on the central and autonomic nervous systems and on another enzyme in the brain called neuropathy target esterase (NTE) but, unlike carbamates, the bond to the enzymes is much stronger and the actions can be irreversible.
At high exposure levels that may be from a single large exposure or repeated low-dose exposures, other enzymes may also be inhibited.
Some of these chemicals are converted in the body to more toxic forms, increasing the effects.
Although OPs are supposedly screened to ensure that the more dangerous forms are not used in agriculture evidence given by experts in court 21 suggest that the testing system is flawed and that some compounds in common use are capable of causing serious long-term damage to the nervous system, including demyelination of the nerves as is found in Multiple Sclerosis.
As mentioned above the OPs are also known to disrupt the vital energy transfer processes required for every cell and process in the human body, inhibiting oxidative phosphorylation.
DNA is itself classified as a natural organophosphate 22 and it has been known for many decades that OP chemicals can damage both DNA and RNA. 12
Glyphosate and glufosinate ammonium are also organophosphorus compounds and although it is claimed that these chemicals are "safe" it is clear that glyphosate adversely affects the mitochondria 23 and scientists have demonstrated that it also inhibits cholinesterase.24
Glyphosate has been found to be implicated in lymph cancer and such cancers are recognised in agricultural workers using organophosphate insecticides in grain stores in the USA. 25
Exposure to organophosphates has also been shown as a cause of persistent asthma. 26
Absorption of organophosphates is by inhalation, ingestion or through the skin, and with all routes severe poisoning may follow.
Contaminated clothing offers a serious risk of poisoning both to the wearer and to any medical staff in attendance.16
Symptoms depend on the actual formulation, the extent of exposure, the route of exposure, the health of the individual exposed, and the length of time of the exposure.
Some of the more severe symptoms may be delayed and with regular use the symptoms may not be realised until it is too late and serious damage has been caused.
The toxin is irreversible in action and accumulative in effect, making this group of pesticides particularly dangerous.
Symptoms initially include exhaustion, weakness, mental confusion, and nausea and could be missed by regular users.
Further exposures can result in abdominal cramps, sweating, increased saliva, running nose and streaming eyes, tightness of chest and constriction of the pupils.
Muscular twitching begins in the eyelids, tongue, face and neck and muscular weakness and convulsions may follow. Both rapid and slow heart rates have been reported and arrhythmias are a common late feature. Diarrhoea, incontinence, ataxia (incoordination, clumsiness, eye movement and speech abnormality), muscle twitching, confusion, cyanosis, respiratory failure, coma and death may follow.
In Chronic poisoning cases neuro-behavioural disorders, cardio-respiratory, and gastro-intestinal symptoms are common. Deterioration of bones, chemical sensitivity, nerve and myelin sheath degeneration and progressive paralysis have been reported. Specific abnormalities of vision have long been suggested as diagnostic for chronic poisoning. 27
Sugar and creatine kinase levels in the blood may be abnormal and fever without infection has been noted.
Repeated exposures at levels below those causing acute poisoning may cause persistent weakness and anorexia.
OP poisoning can result in several recognised "syndromes". 28
Delayed Neuropathy can appear one or two weeks after exposure and presents with peripheral neuropathy, paraesthesia in the toes and feet, fatigue and cramp. The gait is affected and the problems may persist for months or even become permanent.
Chronic neuro-behavioural problems have been widely reported and the symptoms include depression, irritability, confusion, chronic tiredness, headaches and dizziness.
Memory, language, writing, vision, and other cognitive problems have been reported.
Treatment in acute cases is to maintain vital signs and activated charcoal may be used when the poison has been ingested.
The antidote is atropine and pralidoxime mesylate can be administered in attempts to limit the damage caused by the organophosphates, providing that the enzyme damage is not in the "aged" form.
Medical staff must take care to avoid exposure to contaminated clothing or bodily fluids because of the risk of poisoning.
Diazepam can be used to control tremors or convulsions.
There do not appear to be any firm and recommended treatments for chronic OP poisoning and in the majority of cases the sufferers are left to fend for themselves, or to face dubious and often dangerous treatments by those who imply that the serious symptoms are psychiatric in origin.
Laboratory confirmation of acute poisoning is by testing cholinesterase levels in blood and plasma although the results are more useful if pre-exposure levels are known. Metabolites of OPs may be found in the urine and offer evidence of exposure rather than an aid to diagnosis. Neurological testing such as the "Jitter Test" 29 has been shown to demonstrate nerve damage years after the initial exposure. Cognitive defects have been found using psychological testing and blood serum tests may demonstrate brain and neurological damage, indicated by the presence of antibodies, many years after exposure. 30
Paraquat
The toxic effects of paraquat are the result of the salts forming free oxygen radicals that may cause cell damage in addition to irritation to the eyes, mucous membranes, gastrointestinal tract and skin.
Most poisonings are the result of ingestion with only low levels of absorption via inhalation or skin.
Because of the dangers of paraquat pesticide formulations contain chemicals designed to induce vomiting and so nausea and vomiting are early signs. Abdominal pain and diarrhoea may follow with sore ulcerated mouth and throat and difficulty in swallowing and speaking. In severe poisoning malaise, kidney and liver damage may develop at up to 3 days after exposure. Abnormal lung function, lung damage, multi organ failure, convulsions and death may follow up to 3 weeks later.
The majority of severe exposure cases are fatal
Skin contact may result in blistering and ulceration. The nails may be shed in some cases. If the eyes are affected healing may be slow and specialist treatment may be required.
There is no antidote for paraquat poisoning.
Treatment is to prevent further absorption of the chemical, replace fluid loss, and to maintain life. Administration of oxygen may actually worsen the poisoning effects of paraquat.
The compound can be detected in stomach contents and urine.
Phenoxy Herbicides
These compounds are acidic and, like dinitro compounds, they uncouple oxidative phosphorylation.
Exposure is mostly by ingestion but poisoning can also result from inhalation, which can in turn result in systemic toxicity.
Symptoms include burning in the mouth and throat, nausea, vomiting, abdominal pain, facial flushing, sweating, fever and unconsciousness. Hypoglycaemia, hypotension, and excess fluids in the lung may follow. Kidney failure and myopathy may be complications.
Treatment involves administration of oxygen and there is some evidence that intravenous fluids to produce alkalinity in the blood can assist in removing the chemical from the body.
The solvents in the formulations create their own risks.
The chemical can be detected in the blood with the correct equipment.
Phosphine
The toxicity of phosphine is not well understood but it is known to inhibit enzymes such as cholinesterase and to destroy red blood cells in experimental situations.
Phosphine is in a gas form and exposure is therefore by inhalation but aluminium and magnesium phosphides that are used to generate phosphine in the presence of water or water vapour can be absorbed by ingestion.
Phosphine may be produced accidentally when organophosphorus pesticides come in contact with acid and metal or alkali, reacting to produce hydrogen. The action of hydrogen on phosphorus liberates phosphine.
Symptoms include headache, vertigo, loss of balance and incoordination (including speech and eye movements), nausea, vomiting, breathlessness with tight chest and increased secretions, cough, thirst, drowsiness, stomach pain and palpitations. Some of the effects may be delayed for 2 or 3 days but when ingested cyanosis, heart irregularities, liver damage and kidney failure may follow.
Treatment is as an emergency and the patient should first be removed from the contaminated area and taken to hospital to sustain life. There is no antidote but high concentrations of oxygen, and the administration of steroids may be required.
Measurement of plasma and red cell cholinesterase levels may be useful in severe poisoning but there is no specific test for phosphine.
Pyrethrins and Pyrethroids
Nervous system poisons with direct action on the sodium channels in the central and peripheral nervous systems.
In the 1800s it was known that these were irreversible and cumulative nerve toxins 8 and it has long been suggested that their common use would result in serious risk to human health.
These compounds may be absorbed through the skin or respiratory tract but, although they are extremely dangerous to aquatic life at very low contamination levels, they are said to present little risk from ingestion by humans.
It is not known if that claim holds true for commercial formulations that contain solvents.
Pyrethrins are nervous system toxins and are known to induce sensitisation and aggression.
Type I Pyrethroids are nervous system toxins and Type II Pyrethroids cause paresthesias (abnormal sensations), block sodium channels and inhibit GABA pathways.
Symptoms include salivation, tremor, convulsions, hypersensitivity, tingling of the skin, running nose and streaming eyes. Skin allergy has also been reported. In severe poisoning headache, dizziness, anorexia, muscle twitching, convulsions, and coma may occur.
Treatment other than skin decontamination is not usually necessary but in severe poisoning normal methods to sustain life may be required together with drugs to control convulsions.
Measurement of these compounds in bodily fluids is not easy unless poisoning is severe. Metabolites can be measured in urine samples for monitoring purposes.
Triazine Herbicides
Compounds such as Atrazine are reported to be disruptors of hormones and concern has been raised regarding levels in waterways. However the mechanism of toxic action is not known.
Skin absorption rarely results in poisoning but inhalation and ingestion may be significant routes of exposure that may lead to poisoning.
Symptoms are non-specific and may in severe cases result in nausea or vomiting. Some animals such as dogs are especially at risk of poisoning.
Referral to hospital is rarely necessary but in severe cases treatment is of the symptoms.
Triazines and their metabolites can be measured in body fluids but such measurements are of little clinical value.
Urea Herbicides
These compounds can be metabolised in the body to compounds that are oxidants of haemoglobin.
Ingestion is the greatest risk but the compounds can also cause damage when inhaled.
Cyanosis, which does not resolve on administration of oxygen, and chocolate-brown discolouration of blood may lead to anaemia, unconsciousness, convulsions and acute kidney failure.
Treatments are complex and may include emptying of the stomach, administration of oxygen, dextrose, insulin, and in severe cases blood transfusion.
In the UK government's paper on Industrial Diseases it is stated that the symptoms of organophosphorus poisoning are "common in the general population". 3
Most of the published concerns regarding the dangers of pesticides in food have been in regard to residues from applications on the growing crops, often months before harvest. Of greater concern is the fact that Governments have, for decades, approved the deliberate addition to food grains for human consumption, including wheat, oats, barley and rye, of effectively uncontrolled quantities of organophosphates, organochlorines, and recently pyrethroids, as a means to control insect pests in stored grain AFTER harvest.
This is often in addition to residues already in the grain from pesticide applications during the growing season, including pre-harvest glyphosate treatments.
The rules on insect infestation have all but forced farmers to treat the grain with chemicals if it is to have a sale value in the market place. Some farmers have been forced to pay for treatment of grain before the buyer's will accept it for processing 32 and it has been suggested that the presence of the chemical actually protects the finished flour, biscuits, etc., from later infestation. It is unlikely that the forced treatments concern only the particular consignments of grain because the buyer could reject infested grain anyway and the insects, if present, would remain in the grain after treatment.
Some of those chemicals alter protein formation in the human body. 33
Quote “Proteins carry information in their shape and chemical structure: change either of these and you change the information carried by the protein…… Nature’s favourite way of changing the shape or chemical structure of a protein is to stick a phosphate group onto its surface or to remove one…… For phosphates involved in command chains, a phosphate tag often acts as an on/off switch…… It turned out that flipping the Ras switch to the “on” position kick-starts just such a kinase chain……Mutations that jam Ras in the “on” position contribute to the uncontrolled division of cancer cells” 34
The chemicals used in the last 50 years as "undeclared additives" in the food eaten by the entire population include the organochlorine DDT; the organophosphates Malathion, Pirimiphos-methyl, Chlorpyrifos-methyl, Etrimfos; and the pyrethroid, Bifenthrin. They are often used in combination as was Malathion with DDT and currently Malathion with Bifenthrin.
Inevitably grain treated by one farmer with one or two of these products will be mixed with grain treated by different chemicals taken from other farmers during central storage and processing.
No scientist knows what the toxic potential of such mixes can be - especially when those chemicals are heated during processing.
Given the real and ongoing concerns about residues in food resulting from crop spraying, and the damage to health that science suggests such residues induce, it is surprising that these dangerous chemicals were ever permitted for use as additives to food.
It is equally surprising that they remain in general use, given the increasing scientific evidence that safety data is flawed and that very low exposures can cause adverse health effects in humans.
Should anyone be surprised that much of the population suffers with the known symptoms caused by pesticides when we are all exposed to them? .
References:
Dated 01/04/2006